Childhood Leukemia
Prognosis
Prognosis is an estimate of the chance for cure. It helps determine how aggressive the treatment needs to be to have the best chance for cure with the least chance of late effects. The appropriate treatment for each child with ALL is determined by analyzing several features related to the child and to the leukemia cells.
I don’t think hearing prognosis numbers helps. I actually asked them not to tell me those numbers because I wanted to hope. I knew my child’s type of ALL was rare and many children don’t survive. They told me anyway, and I found those numbers to be a huge barrier I had to scale to find my hope again. We’re all different—I know parents who want the prognosis numbers. But I didn’t.
Below are some of the factors doctors consider when determining prognosis. For more detailed information about prognostic factors, you can visit the National Cancer Institute (NCI) Physician Data Query website at www.cancer.gov/types/leukemia/hp/child-all-treatment-pdq#section/_22.
- WBC count at diagnosis: The WBC count at diagnosis helps predict response to treatment. Children with a low WBC count (<50,000) usually have a more favorable prognosis, and therefore need less intensive therapy than children with higher WBC counts.
- Age: Children ages 1 to 9 typically do better than infants, older children, or teens. As a result, more aggressive treatments are usually needed for infants and children older than age 9.
- Gender: Overall, boys have a slightly worse prognosis than girls. Possible reasons are that more boys are diagnosed with T-cell ALL, only boys have testicular relapses, and fewer boys have extra chromosomes (called hyperdiploidy) in their cancer cells.
- Response to treatment: One factor in a child’s prognosis is how quickly the leukemia cells disappear after starting treatment. Treatment is divided into phases, the first of which is called induction. At the end of the induction phase, bone marrow is sent to a specialized laboratory to see whether any blast cells are present. This measure of residual leukemia is called minimal residual disease (MRD). If there has been a rapid reduction of blasts in the marrow, the child may need less intensive treatment.
Nico’s white blood cell (WBC) count at diagnosis was relatively low (15,200 μL), and his cytogenetics were favorable (trisomy 4, 10). The week of diagnosis, our oncologist only discussed the low- and standard-risk treatment courses with us. We were shocked when the combination of day-8 peripheral blood and day-29 bone marrow MRD bumped our son into the high-risk category. It was like getting the diagnosis all over again. I had deluded myself into thinking that the low and standard category provided some safety net, which was really never true. The fact is some children in the low- and standard-risk protocols run into problems, while some children in the high- and very high-risk protocols avoid complications. I will admit that I am still jealous when I look at the standard protocol. But as someone once counseled me, “Be grateful that your child’s risk category was properly identified. This enables us to adjust his treatment to give him the best chance of success.”
-
CNS status at diagnosis: If ALL is suspected, a sample of the cerebrospinal fluid (CSF) is obtained during a lumbar puncture (also called spinal tap). Children without leukemia blasts in the CSF at diagnosis have a better prognosis than those who do. The results of the first lumbar puncture at diagnosis will be described as:
- CNS1: No leukemia blasts found in CSF (76% of children)
- CNS2: Fewer than five leukemia blasts (per μL) found in CSF (19% of children)
- CNS3: Five or more blasts (per μL) found in CSF (5% of children)
-
Number of chromosomes: Normal cells contain 46 chromosomes (22 pairs and the sex chromosomes—XX for females or XY for males).
- Hyperdiploidy: Some leukemia cells contain extra copies of entire chromosomes, giving them more than 46 (called hyperdiploidy). Approximately 20 to 25% of children with B-cell ALL have 51 to 65 chromosomes per cancer cell. Although the prognosis of children with hyperdiploidy is favorable, factors such as age, WBC count, and early response to treatment also affect prognosis. Hyperdiploidy is uncommon in children with T-cell ALL.
- Hypodipoidy: Around 6% of children with B-cell ALL have fewer than 44 chromosomes in the leukemia cells. This condition is called hypodiploidy and carries a poor prognosis.
- Extra copies of particular chromosomes: Children with extra copies of chromosomes (e.g., chromosome 4 or 10) have an especially favorable prognosis. These extra chromosomes are called trisomies.
When Liza was diagnosed, our oncologist told us that at the initial bone marrow aspiration he would be able to determine if she had leukemia or not, and then the other sample would go for cytogenetic testing. It took about 15 days to get the results back and Liza had hyperdiploidy, with triple trisomies 4, 10, 17.
-
Chromosome translocations (swaps between chromosomes) or rearrangements (genes swap places on the same chromosome): Translocations and rearrangements are extremely common in B-cell ALL, and some of these are known to affect prognosis.
- Children with the ETV6-RUNX1 gene fusion (also known as TEL/AML1) need less intensive therapy unless MRD is positive at the end of induction.
- The Philadelphia chromosome, called t(9;22) or Ph+, is found in approximately 3% of children and teens with B-cell ALL (see Figure 3–2). It is associated with a poor prognosis, especially in older children with high WBC counts who have a slow response to treatment. This translocation is found more often in teens and young adults with ALL. Newer treatment protocols that include a tyrosine kinase inhibitor (TKI) such as imatinib have dramatically increased survival rates for children and teens with Ph+ ALL.
- Translocations and rearrangements involving the MLL gene are only found in 5% of children with ALL, but they are in up to 80% of infants with ALL. The most common MLL translocation is t(4;11). It occurs most often in infants with leukemia cells in the CNS and high WBC counts at diagnosis. It is associated with a poor outcome in infants but not in children with T-cell leukemia.
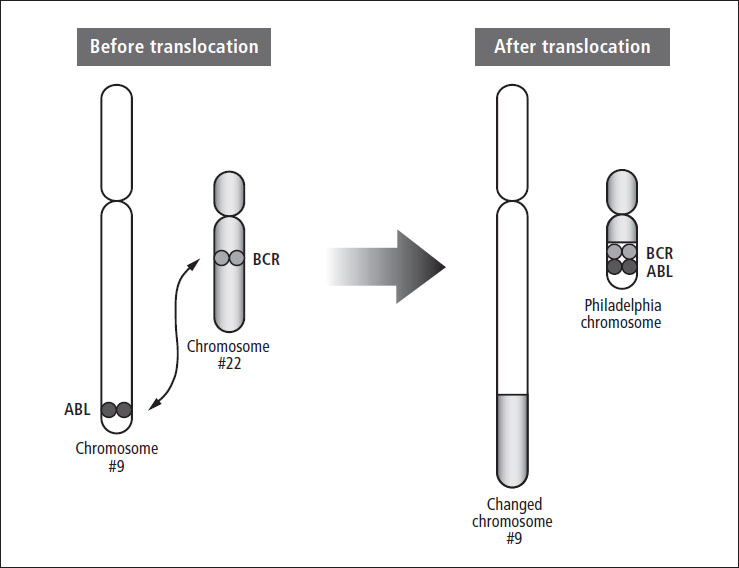
Figure 3–2: The Philadelphia chromosome
My 13-year-old son had been sick with ear and other infections for a few months. Then he started having pain in his legs and pelvis but we thought it was from growing, running track, and playing basketball. The pain got worse and he went to physical therapy for eight weeks. One night, it became unbearable and we went to the ER. When he took off his shirt, I saw that his back was covered by petechiae, which was new. They drew labs, took him off to x-ray, and the next thing I knew, a doctor and a chaplain came in and said his bone marrow was the cause of the pain and that we needed to go up to the pediatric oncology floor, where he was diagnosed with ALL. That was Tuesday. On Friday, we learned it was Ph+ ALL, which changed everything, including his chance for survival.
Different institutions and research groups use different criteria to determine prognosis and plan the best treatment. Most groups divide children with ALL into four categories—low risk, standard risk, high risk, and very high risk—based on some combination of the following:
- Age at diagnosis
- WBC count at diagnosis
- Whether the leukemia cells are B lymphocytes or T lymphocytes
- Presence of leukemia blasts in CNS at diagnosis
- Presence of leukemia blasts in the testes
- Certain changes in chromosomes or genes
- Number of chromosomes in leukemia blasts
- Response to treatment (number of leukemia blasts in the peripheral blood on day 8 and bone marrow MRD on day 29)
I focused on prognosis in an unhealthy way after my daughter was diagnosed. I kept asking the doctors why she was at a high risk for relapse. I sort of became consumed with hearing all of the prognostic percentages and where they came from. Our attending sat me down at one point and said that the prognosis was more for the doctors to determine the best treatment than it was anything to do with my individual child. When he said it was either 100% or 0% chance for us because my daughter would either live or die, something clicked and I stopped worrying about the prognosis numbers. Now that we are several years out, I so value his wisdom. I’ve known kids with very good prognoses who relapsed, and I know kids with really poor diagnoses who tolerated treatment well and are doing great many years later.
Table of Contents
All Guides- Introduction
- 1. Diagnosis
- 2. Overview of Childhood Leukemia
- 3. Acute Lymphoblastic Leukemia
- 4. Acute Myeloid Leukemia
- 5. Juvenile Myelomonocytic Leukemia
- 6. Chronic Myelogenous Leukemia
- 7. Telling Your Child and Others
- 8. Choosing a Treatment
- 9. Coping with Procedures
- 10. Forming a Partnership with the Medical Team
- 11. Hospitalization
- 12. Central Venous Catheters
- 13. Chemotherapy and Other Medications
- 14. Common Side Effects of Treatment
- 15. Radiation Therapy
- 16. Stem Cell Transplantation
- 17. Siblings
- 18. Family and Friends
- 19. Communication and Behavior
- 20. School
- 21. Sources of Support
- 22. Nutrition
- 23. Insurance, Record-keeping, and Financial Assistance
- 24. End of Treatment and Beyond
- 25. Relapse
- 26. Death and Bereavement
- Appendix A. Blood Tests and What They Mean
- Appendix B. Resource Organizations
- Appendix C. Books, Websites, and Support Groups

